Release Of Energy From Food
NOTIFICATIONS
The foods nosotros eat supply the energy needed by the trunk to drive its complex chemic, mechanical and electrical systems. Where does this energy come up from, how is it locked into food molecules and how is it released?
Energy from the Dominicus
The energy content of all food molecules can exist traced dorsum to the Dominicus. It is the procedure of photosynthesis that locks the Sun's energy into unproblematic carbohydrates like glucose. Although the photosynthetic process, which is carried out in the chloroplasts present in plant cells, involves numerous steps, it can exist summarised in the following equation.
| Free energy from sunlight trapped by chlorophyll present in the chloroplasts | + | 6CO2 carbon dioxide | + | 6H2O water | → | C6H12O6 | → | 6O2 oxygen |
This reaction only gain with an input of solar energy, and the production glucose stores this free energy as chemical potential energy.
Plants are able to convert some of the glucose formed into starch and other macronutrients such as proteins and lipids. The origin of the energy locked into these molecules is from the Sun.
Releasing the locked-up free energy
The food nosotros swallow supplies the body with energy-rich molecules like glucose. On inbound the cells of the torso, these molecules are broken downwardly in a series of steps to reform carbon dioxide and water, releasing free energy to be used by the body.
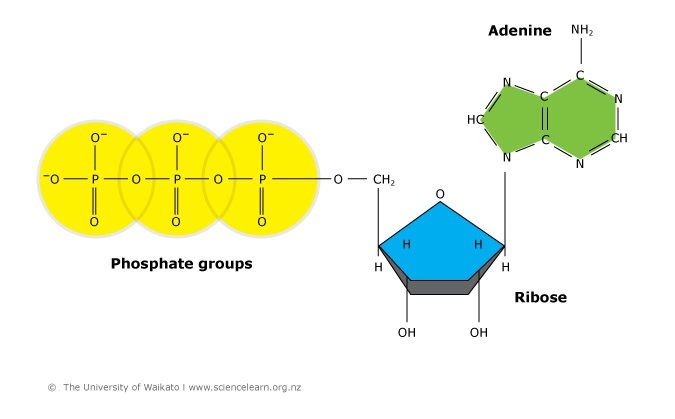
Some of the chemic potential free energy locked into these molecules is transferred within the cell to a substance chosen adenosine triphosphate (ATP).
ADP + P + energy → ATP
ATP is oftentimes referred to as the free energy currency of the cell as it is used to drive the torso'southward circuitous chemic, mechanical and electrical systems.
This energy transfer pathway, which occurs within all torso cells, is called aerobic respiration, and for carbohydrates similar glucose, information technology is finer the reverse of photosynthesis.
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
The amount of energy released for each glucose molecule consumed produces between 36 and 38 ATP molecules.
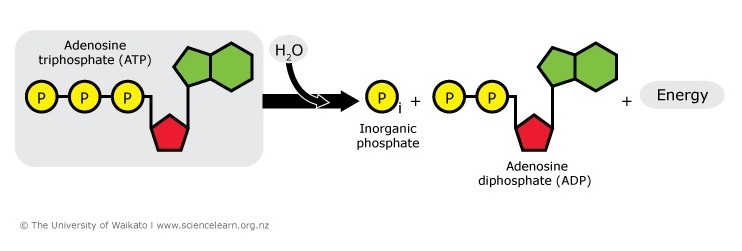
When the cell needs free energy to drive its circuitous chemic, mechanical and electrical systems, ATP breaks downwardly, releasing the required energy.
ATP → ADP + P + free energy
Efficiency of aerobic respiration
Not all of the free energy locked into glucose through photosynthesis is released by aerobic respiration. Several different processes are involved, each of which consists of several steps. This results in some of the free energy beingness converted into heat and not ATP.
The energy conversion efficiency for glucose lies between 38–44%, depending on the cell type. In terms of the trunk as a automobile, this compares very well with the xx–25% efficiency of well-nigh machines.
Counting the free energy of foods
The standard energy unit of measurement is the joule. It is divers in the following way: 4.eighteen joules is the energy needed to rut 1g of water by 1°C.
The joule is a small unit for everyday purposes, so in food chemistry, the kilojoule is the preferred unit (1000J = 1kJ).
The macronutrients in the foods we consume accept been analysed for their bachelor free energy content when metabolised in the body. The energy values calculated are:
- carbohydrate – 17kJ/g
- protein – 17kJ/one thousand
- fatty – 37kJ/g.
If any given repast is carefully analysed for its macronutrient limerick, it is possible to summate the energy input it gives.
Here is an example:
| Principal meal items | Portion size (g) | Energy (kJ) | Protein (g) | Fat (thou) | CHO (g) |
|---|---|---|---|---|---|
| Roast irish potato | 200 | 866 | 5.0 | ane.0 | 44.0 |
| Chicken (roast) | 150 | 1010 | 36.ix | 10.4 | 0.0 |
| Asparagus | 80 | 88 | 2.8 | 0.two | 2.0 |
| Carrot | 50 | 55 | 0.iv | 0.1 | ii.8 |
| Butter chicken sauce | 40 | 240 | 1.0 | 4.0 | 3.9 |
| Ice cream (depression fat) | 120 | 706 | v.eight | four.0 | 29.half-dozen |
| Peaches (canned) | 120 | 270 | 1.1 | 0.12 | 15.0 |
| Orange juice | 250 | 364 | 1.9 | 0.3 | 21.5 |
| Total | iii,599 |
(Guess values based on the Concise New Zealand Food Composition Tables eighth edition 2009).
The full daily intake for a moderately active, healthy adult is, on average, about 8,700kJ. Other meals such equally breakfast and lunch along with 'in betwixt' snacks supply the rest of the daily energy needed.
Useful link
The Concise New Zealand Nutrient Composition Tables provide upward-to-engagement information from the New Zealand Food Composition Database in PDF and Microsoft Office Excel file format. The Concise Tables comprise information on key nutrients for commonly consumed foods and are ideal for quick reference.
Would you similar to have a curt survey?
This survey volition open in a new tab and yous tin can fill it out after your visit to the site.
Release Of Energy From Food,
Source: https://www.sciencelearn.org.nz/resources/1833-unlocking-the-energy-in-foods
Posted by: rodriguezwrearpon76.blogspot.com



0 Response to "Release Of Energy From Food"
Post a Comment